Section
5
Assessing Adiposity
Air Displacement Plethysmography (ADP)
The ADP method uses the volume of air displaced by a participant in a sealed testing chamber to measure the participant’s body volume and estimate body density.
Predictive equations are then used to estimate values for total FM and FFM. The ADP technique can be used to measure infants beginning at birth through approximately 6 months weighing up to a maximum weight of 8 kg (using the PEA POD instrument) and to measure individuals aged 6 years and older and weighing between 35 and 200 kg (using the BOD POD instrument). However, there is a pediatric adaptor for testing young children between the ages of 2–5 years.95
Procedure
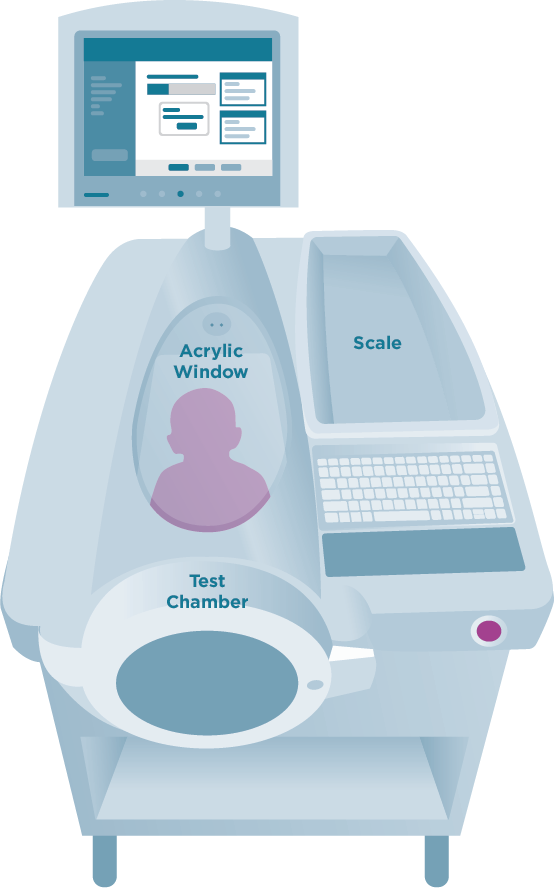
Measurement using ADP requires availability of commercial equipment from a sole manufacturer (Cosmed Inc, Concord, CA). The PEA POD is used in infants up to a maximum weight of 8 kg (approximately 6 months) and the BOD POD for persons aged 6 years and older and weighing between 35 and 200 kg. The BOD POD can be fitted with a pediatric adaptor seat for use in children aged 2–5 years95 (Figure 12).
Figure 12: BOD POD Fitted with a Pediatric Adaptor Seat
The BOD POD system is stationary and consists of a front test chamber and a rear reference chamber in a closed system. The participant sits in the front test chamber while the test is conducted. Participants are required to wear a tight-fitting bathing suit or tight-fitting undergarments, and an acrylic cap that covers the head so that trapped air within the hair is minimal. Loose-fitting clothes, scalp hair, and facial hair can introduce error such that percent fat is underestimated.96 Participants should have fasted for at least 2 hours and emptied their bladders immediately before the test. Participants are first weighed on the BOD POD weight scale, and height is measured using anthropometric techniques described in this guide. In addition to the values for body mass and height, the age of the participant is entered into the software algorithm of the instrument before the test is conducted. The participant sits motionless in the BOD POD test chamber with the door closed while the test is performed. Air is gently blown into the testing chamber while the participant breathes normally. Body volume is derived from the ratio of the pressure in the reference and test chambers based on Poisson’s law, which states that volume varies inversely with pressure when temperature is constant.97 A diaphragm mounted on the common wall between the chambers oscillates during testing under computer control. When the volume is increased in one of the chambers, it is decreased by the same amount in the other chamber and vice versa. The pressure in each of the two chambers responds immediately to this volume change or perturbation by the participant sitting in the chamber, and the magnitude of the pressure changes indicates the relative size of each chamber. The difference in the change in air pressure when the chamber is occupied by the participant can be used to estimate body volume, or the volume of air the participant displaces. The test also requires an estimate of lung gas volume of the participant. This can be estimated using predictive equations that are based on height and age or can be measured directly during the BOD POD testing. If measured directly, the participant is asked to perform a lung volume measurement, which requires that he or she breathe through a disposable tube with gentle puffs of air when cued by the technician. Pressure in the breathing tube changes as the subject’s diaphragm contracts and expands, which, combined with BOD POD chamber pressure changes, allows for an estimation of the participant’s lung volume. Two lung volume tests are performed on a participant, and the volumes are averaged when the values are within 150 mL.98 The total time to measure body volume while sitting in the closed chamber is about 2 minutes. Results are displayed on the computer monitor. Quality control is conducted regularly. The BOD POD weight scale must be calibrated daily using a certified 20 kg National Institutes of Standards and Technology (NIST) weight. In addition, before each individual participant is tested, a calibration test is performed using a standard 50.218-liter calibration cylinder placed in the empty BOD POD chamber.
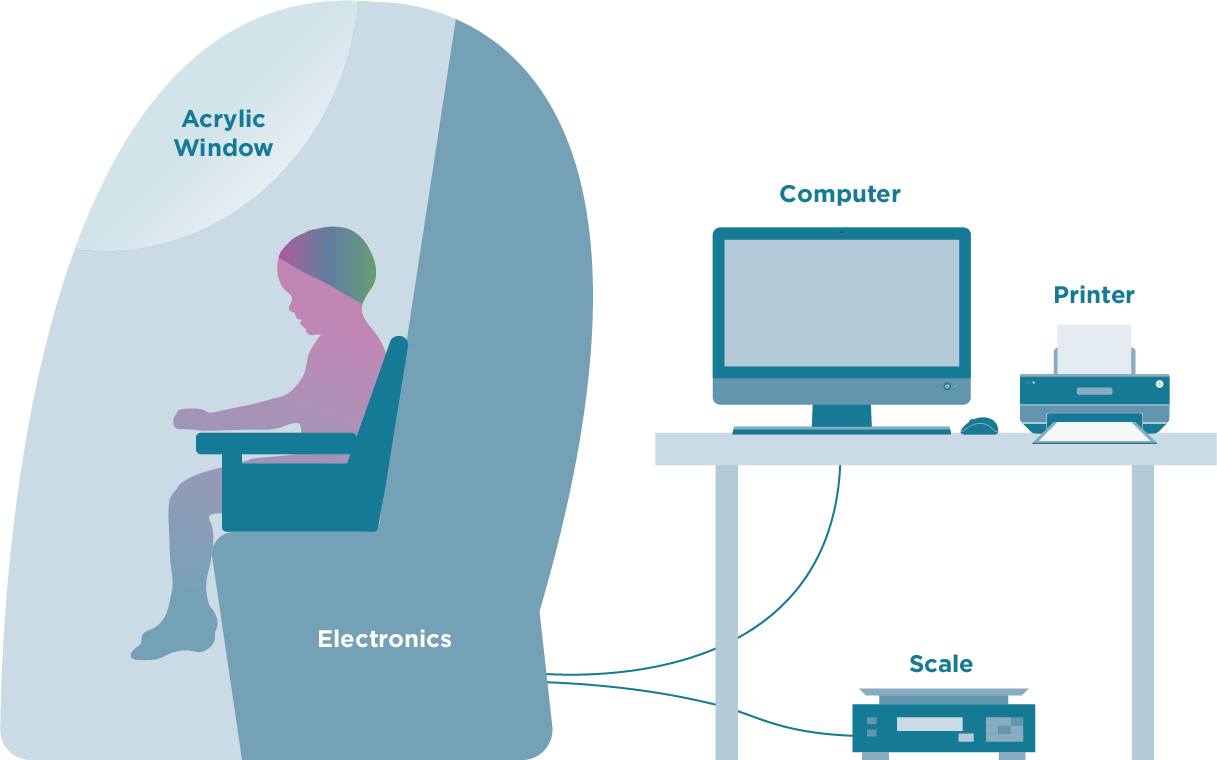
Figure 13: PEA POD
The PEA POD system measures body volume in a similar fashion to the BOD POD (Figure 13). For the PEA POD, a movable cart houses the reference chamber and calibration volume, which allows it to be brought to the infant bedside in hospital settings. The test chamber along with a weight scale are mounted on the cart’s top surface. A volume-perturbing diaphragm is located between the test and reference chambers, and a pneumatic valve (calibration valve) allows the test chamber to be connected to the calibration volume.99 Infant length and weight are first measured using the previously described anthropometric techniques. These values and age are entered into the software before the testing is conducted. The ADP testing is conducted with the infant naked, except for a nylon head cap that is used to remove the effect of trapped air among the hairs. Alternatively, the hair can be smoothed flat using oil. The infant is then positioned on a tray that slides into a sealed testing chamber with a clear acrylic covering. When the chamber door closes, pressure changes are measured over a 2-minute period. The test ends with the door opening automatically, and results are displayed on the computer monitor. Similar to the BOD POD, before each measurement, a volume calibration is run using a certified aluminum cylinder (5 L), and the weight scale is calibrated using a certified NIST weight (5 kg) daily.
Estimates of Body Fat, Interpretation, and Limitations
Percent body fat is estimated by prediction equations in the software that use body density, which is calculated using measured body volume and body weight of the participant (e.g., body density is the ratio of the body’s weight (mass) to body volume). In this approach, the densities of FM (0.9007 g/cm3) and FFM (1.100 g/cm3) are assumed to be the same across all adults, irrespective of age, race/ethnicity, and disease. For children, the densities of FM (0.9007 g/ml) are assumed constant, and age- and sex-specific FFM density coefficients are used.10
Limitations of this method include the assumption that the density of FFM is stable irrespective of age, race/ethnicity, and disease and that lung volume is correctly estimated. Age- and sex-specific density of FFM constants applied may not be appropriate for all infants due to the rapid changes in body water in the early weeks of life and in some diseased states with abnormal body water status.
Validity and Reliability
The ADP has been validated for use in infants from birth to about 6 months and in children aged 6 years and older. However, a gap remains for the use of ADP in children from 6 months through 5 years of age. For adults, the accuracy of percent body fat measures is within –4.0% to 1.9% when ADP is compared to under-water weighing.98,100–102 Comparing ADP to DXA (discussed below), the accuracy of percent fat measures are between –3.0% and 1.7%.98,102,103 Between-day test-retest correlation coefficients for body density and percent fat using the ADP are 0.95 in adults and 0.90 in children.100
…it is important to note that the ADP was not well tolerated among children aged 1–5 years (useable data were obtained on 31 of 74 (42%) children enrolled), thereby limiting its use.
A recent comprehensive review by Mazahery et al., provides a detailed discussion on PEA POD in full-term and pre-term infants.104 PEA POD is reliable for assessing percent fat in full-term infants but has only modest accuracy, with overestimation or underestimation of percent fat by 6% to 8%. Few data exist on the validity of PEA POD in preterm infants; performance appears to be reasonable but with modest accuracy, as summarized by Mazahery.104 The lack of precision of the instrument in young children and especially in children younger than age 5 years may be related to the small body size of younger children within the chamber.105 Although one study reported no statistical difference between percent fat by the ADP with pediatric attachment and percent fat measured by 4-compartment model,106 it is important to note that the ADP was not well tolerated among children aged 1–5 years (useable data were obtained on 31 of 74 (42%) children enrolled), thereby limiting its use. When compared to a multi-compartment model, with or without an adapter designed for pediatric use with this equipment, the BOD POD was shown to have reduced accuracy for use in infants and children younger than 7 years due to compliance issues, which included movement, talking, and crying during the test.106 Reliability was established in 17 infants (1 to 22 weeks) tested three times over two days.107 The within-day reliability for percent fat was 2.85% and the between-day reliability was 2.95%.
Reference Data
National reference data for children in the U.S. population are not available.
Accessibility, Training, and Cost
Accessibility is low as the equipment is expensive and largely located at specialized medical or research centers. All ADP systems are available from a single manufacturer (Cosmed Inc, Concord, CA). The PEA POD is for use in infants up to a maximum weight of 8 kg (approximately 6 months old) and the BOD POD for persons 6 years and older weighing between 35 and 200 kg. A pediatric option is available for assessing body composition using the BOD POD in children aged 2–6 years. The pediatric option accessory includes a customized seat insert, calibration standards, and modified software program, developed in an effort to allow for longitudinal measures from childhood by the same technology.106 Costs are high for initial purchase (BOD POD $50,000; PEA POD $90,000) and an annual service contract per system is required (BOD POD $3,000; PEA POD $1,500). Testers require training, and the level of skill required is moderate.
Acceptability, Participant Burden, and Risk
Acceptability of the ADP method is moderate to high in adults, but data on its acceptability in children are more limited. One study among children aged 1–5 years found its acceptability was low, with a measurement being obtained in less than 50% of these children. Risk is low, and the measure is generally noninvasive. Burden is low-to-moderate. Wearing a tight-fitting undergarment for the test may be a significant source of embarrassment. Fasting may be difficult in children. Movement during the BOD POD test must be minimal, which is difficult for infants and young children. For the PEA POD, excessive crying can result in a failed test. Persons with claustrophobia and larger persons may be unable to tolerate the sealed chamber despite the window. The BOD POD excludes persons weighing >200 kg.
Summary
ADP is a non-invasive, quick, safe method that does not require sedation. The cost is modestly high in comparison to other methods. Training of staff is required, but it does not require specialized technicians. However, the equipment is not portable, and the availability of the equipment is often limited to hospital-based medical and research facilities due to high purchase cost and storage requirements. Participant burden is modest, requiring fasting and wearing tight-fitting undergarments or swimsuits. The need to have minimal movement during testing is a limitation when assessing children younger than the age of 6.